Monday, March 8, 2010
Someone is Finally Listening to Sculptra Damage
The FDA provided me with some of the redacted information. There were over 700 scuptra product technical complaints received by Sanofi during a two year period prior to obtaining the FDA cosmetic use approval. Sanofi reported zero of those events to the FDA as is required by law. With their multinational staff and multi level review they were confuse about what was reportable. Had they reported adverse events as required many of us may not have been harmed. If you look at the FDA maude database for adverse event reporting, Sanofi has a "new decision tree" for determining if something is reportable. Since the beginning of 2010 there have been about 40 new reports of "serious adverse events" using this new decision logic. This Sculptra Damage did not need to happen to us. If you were harmed by this dangerous and unpredictable product to to www.sculptradamage.com there is information on how to report your situation to both the manufacturer and the FDA.
Monday, February 22, 2010
FDA compliance inspection shows Sanofi Adventis failed to report serious adverse events to the FDA
There is much redacted information.
Through Freedom of Information, I was provided with an FDA inspection document FEI 3000719690. This is a compliance inspection report. I cant help but wonder, had Sanofi reported adverse events as required, perhaps the FDA would have required more stringent label warnings and marketing precautions and I might not have been harmed. It is interesting to me that the FDA officials documented that during the time period evaluated by inspectors, 08/20/2007 – 07/31/2009, the only Sculptra adverse events reported to the FDA were not from Sanofi Adventis. The FDA had 4 reports made directly to them, of which one is mine, two are from non-mandatory reporting physicians and one from a sales representative. It is interesting that during this inspection it was discovered that (redacted number) of Product Technical Complaints were reported to the company. The FDA investigators reviewed a random 33 of those (larger number redacted) complaints and found it objectionable that many of these adverse events were not reported to the FDA as required under the terms of P030050 and its subsequent versions. In fact Sanofi Adventis reported zero adverse events to the FDA. Reportedly, Sanofi Adventis, a very well staffed knowledgeable multi national drug company was “confused” about what is and is not reporting criteria. I am sure that the wider marketing strategy did not include reporting adverse events so claiming to be ignorant of reporting criteria helps until the larger market approval is achieved.
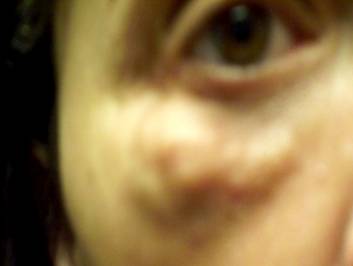
The large inflammatory granulomatous nodules I am experiencing are certainly not "papules" described to the FDA during the hearings. Furthermore I am not alone. Many people have had serious adverse events from Newfill or Sculptra or Sculptra Aesthetic.
I was told "its the same thing that is used in absorbable sutures". Now I have learned that is NOT TRUE. Sutures are a more amorphous form of poly lactic acid which is intended to dissolve. The 100% poly l lactic acid product used in my face is a crystalline form. The company will not tell me how long it stays in my body. From what I have learned, it could be more then 5 years!! With the clumping that took place in my case it could be far longer. How Dare they Lie to the FDA and Lie to me. I am going to be meeting with both Congressman Tonko and Senator Gillebrant to request further intervention. If the company failed to comply with the terms set forth it should be removed from the market until adequate non Sanofi sponsored safety data is available.
Sunday, January 24, 2010
www.sculptradamage.com all updated
Because of endless litigation I have put the website on hold. When everything is over I will share with people the process of litigation and how messed up it is. The web site www.sculptradamage.com was created by a few of us who have had very very bad experiences with the product Sculptra. It has a reading room that provides detailed information on immune system foreign body response and medical and scientific information. It provides information on a few people who have been harmed and offers a forum area for people to post. I must say when someone tells me how great the stuff is, I am very glad they are still OK. Anyone who continues to use this unpredictable product should really look into the literature provided and the experience of people in other countries. New-Fill is no longer legal in some countries where it caused so many problems. I guess it will take someone really important like Tyra Banks or Oprah to get sick from this product before anyone really takes it seriously.
Saturday, January 23, 2010
The mess that sculptra has made of my life
I have not updated this site for a while. I have been pretty depressed. I had surgery to remove hardware from a 1992 injury. The Sculptra BIOFILM infection spread to the other parts of my right eye. I am going to need 2 more surgeries on the right and the left just looks awful. I did manage to get the www.sculptradamage.com website updated. Please have a look.
Subscribe to:
Posts (Atom)